CLINITEK Status® Connect System simplifies wireless or wired connectivity and testing oversight in point-of care urinalysis for improved risk management.
|
Semi-quantitative Tests Measured
|
|
|
Routine urine tests
|
albumin, bilirubin, creatinine, glucose, ketone, leukocytes, nitrite, pH, protein, specific gravity and urobilinogen
|
|
Kidney disease test
|
albumin-to-creatinine ratio
|
|
Pregnancy test
|
Human Chorionic Gonadotropin (hCG)
|
|
General Specifications
|
|
|
Depth
|
27.2 cm
|
|
Width
|
17.1 cm
|
|
Height
|
18.5 cm
|
|
Weight
|
2.3 kg
|
|
Electrical rating
|
100V – 240V AC, 50-60 Hz (with in-line lead)
|
|
Battery operation
|
Not available
|
|
Ambient temperature range
|
18 to 30ºC
|
|
Humidity range
|
18-80% relative humidity, non-condensing
|
|
Compliance
|
UL, CE, EMC
|
|
Calibration
|
automatic, self-calibrating
|
|
Communication Capabilities
|
|
|
Communication protocol
|
HL7, POCT1-A2
|
|
Data transmission options
|
wireless, Ethernet or serial connection
|
|
Interfaces with leading point-of-care
data management solutions* |
|
|
Memory
|
|
|
Patient test results
|
700
|
|
QC test results
|
200
|
|
Operator IDs
|
700
|
|
Quality Control Management Features
|
|
|
QC testing and reporting
|
|
|
QC reminders or mandatory prompt
|
|
|
QC lockout
|
|
|
Barcode Data Entry
|
|
|
Patient ID, operator ID and name,
QC control type, lot number, and expiration date |
|
|
Barcode Symbology Supported
|
|
|
Code 93, Code 39 (with and without check digit), Code 128, Coda Bar, Interleaved 2 of 5 (with and without check digit)
|
|
|
Operator Security Features
|
|
|
Define up to 700 operators
|
|
|
Operator lock-out to prevent unauthorized use
|
|
|
Customize access level for each operator for the following functions:
|
The CLINITEK Status Connect System provides automated reading of the Multistix® family of urinalysis tests:
Auto-Checks:
*Only available when using Siemens Healthineers test strips with IR or color bands
§Point of Care Diagnostic Testing Worldwide Markets. Trimark Publications. June 12, 2009
‡ Not available in all markets. Product availability may vary by country.
Atellica, Auto-Checks, CLINITEK Advantus, CLINITEK Status, DCA Vantage, epoc, Multistix, POCcelerator, RAPIDComm, RAPIDLab, RAPIDPoint, STIX, and all associated marks are trademarks – owned by or licensed to Siemens Healthcare Diagnostics Inc., or its affiliates. All other trademarks and brands are the property of their respective owners.
Service is essential to keep medical equipment operational and safe. Our Nordic service team consists of experienced service technicians stationed across the Nordic countries. Our service technicians are ready to take on your every service need.”

Nasal PAP ventilation system
The SuperNO2VA nasal PAP ventilation system uses the flow from any oxygen source. Delivers positive airway pressure to stent open the upper airway, allowing for the preoperative delivery of positive pressure ventilation and oxygen for patients with a decreased level of consciousness. The SuperNO2VA™ nasal PAP ventilation device is available in medium and large sizes and is offered as a standalone mask with a head strap and as a system, kitted with a hyperinflation bag. These configurations offer flexibility as it allows the SuperNO2VA™ nasal PAP ventilation device to be used with either an anesthesia machine or with only an oxygen flow meter.
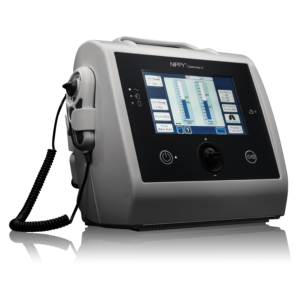
Versatile Airway Clearance
Introducing the Clearway 2 with TreatRepeat®. The second-generation MI-E device by Breas incorporates the latest technology to bring effective and accurate treatment for patients requiring airway clearance and cough assistance. TreatRepeat function allows clinicians to manually deliver different numbers of insufflations and exsufflations and then save the treatment they have just delivered to the device to be repeated automatically. Helping to improve accuracy and speed of titration and set-up.
Multiple mode options
Up to four treatment profiles can be saved to the device.The settings used in the manual and programmed modes are also saved to the device. This allows for up to 7 MI-E prescriptions to be saved. The treatment settings in the IPPB and NIV modes are also saved. The programmable mode enables the delivery of multiple insufflations as part of a cough cycle.

Untethered six-minute walk test
Vyntus™ WALK allows patients to perform an untethered six-minute walk test (6MWT) by wearing wireless sensors that communicate data to a technician-held tablet PC. Standardize testing to the recommended is 6MWT protocol, because consistent testing yields consistent data for comparison
The system is both patient- and technician-friendly and because we know a single sensor does not fit all clinical needs, comes with an array of easy-to-wear sensors. Because standardizing the complete testing process is critical to meaningful data for patient trending, Vyaire has integrated the complete ATS testing standards in the workflow-driven tablet software application.

Portable hospital spirometer
AioCare is a professional system to monitor and treat pulmonary diseases, consisting of a portable hospital-grade spirometer connected to a smartphone app and an online panel to access the results. The device enables a full spirometry test to be conducted in a doctor’s office, including a bronchial challenge workflow. If monitoring needs to go beyond the healthcare system, AioCare may be used for patients at home.