The CLINITEK Status®+ Analyzer features automatic checks (Auto-Checks*). The analyzer automatically checks each test strip for humidity exposure, common sample interferences† and strip identification for Siemens Healthineers test strips. Together, these provide improved clinical information.
|
Semi-quantitative Tests Measured
|
|
|
Routine urine tests
|
albumin, bilirubin, creatinine, glucose, ketone, leukocytes, nitrite, pH, protein, specific gravity, and urobilinogen
|
|
Kidney disease test
|
albumin-to-creatinine ratio
|
|
Pregnancy test
|
Human Chorionic Gonadotropin (hCG)
|
|
User Interface |
|
|
Touchscreen
|
|
|
Computer Interface |
|
|
Uni-directional via Serial Port (RS232)
|
|
|
Data Entry |
|
|
Onboard touchscreen
|
|
|
Power Requirements |
|
|
Electrical rating
|
100V – 240V AC, 50-60 Hz (with in-line lead)
|
|
Battery operation
|
6 AA batteries, non-rechargeable
|
|
Dimensions/Weight |
|
|
Depth
|
27.2 cm
|
|
Width
|
17.1 cm
|
|
Height
|
15.8 cm
|
|
Weight
|
1.66 kg
|
|
Operation |
|
|
Temperature range
|
18 to 30ºC (64 to 86ºF)
|
|
Humidity range
|
18-80% Relative Humidity, non-condensing
|
|
Calibration |
|
|
Automatic, self-calibrating
|
|
|
Memory |
|
|
Patient test results
|
700
|
|
QC test results
|
0
|
|
Operator IDs
|
700
|
Easy to Use and Convenient
The CLINITEK Status+ Analyzer provides automated reading of the Multistix® family of urinalysis tests:




*Only available when using Siemens Healthineers test strips with IR or color bands
§Point of Care Diagnostic Testing Worldwide Markets. Trimark Publications. June 12, 2009
‡ Not available in all markets. Product availability may vary by country.
Atellica, Auto-Checks, CLINITEK Advantus, CLINITEK Status, DCA Vantage, epoc, Multistix, POCcelerator, RAPIDComm, RAPIDLab, RAPIDPoint, STIX, and all associated marks are trademarks – owned by or licensed to Siemens Healthcare Diagnostics Inc., or its affiliates. All other trademarks and brands are the property of their respective owners.
Service is essential to keep medical equipment operational and safe. Our Nordic service team consists of experienced service technicians stationed across the Nordic countries. Our service technicians are ready to take on your every service need.”
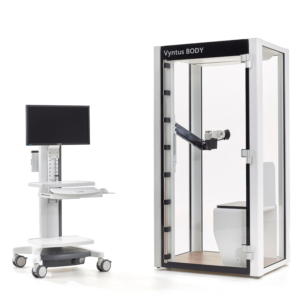
Vyntus™ BODY Plethysmograph
People come in all shapes and sizes, so we designed Vyntus BODY to accommodate the most patients without increasing the cabin footprint. Many enhancements were incorporated to make Vyntus BODY accessible and user friendly for both patients and technicians.
Vyntus BODY employs much of the same breakthrough technology found in the Vyntus ONE, but with a redesigned breathing circuit and our new Ultrasonic Sensor Technology.
In addition, Vyntus BODY is enhanced with additional innovation such as digital pressure compensation, which helps to reduce environmental influences and improves measurement accuracy.

Untethered six-minute walk test
Vyntus™ WALK allows patients to perform an untethered six-minute walk test (6MWT) by wearing wireless sensors that communicate data to a technician-held tablet PC. Standardize testing to the recommended is 6MWT protocol, because consistent testing yields consistent data for comparison
The system is both patient- and technician-friendly and because we know a single sensor does not fit all clinical needs, comes with an array of easy-to-wear sensors. Because standardizing the complete testing process is critical to meaningful data for patient trending, Vyaire has integrated the complete ATS testing standards in the workflow-driven tablet software application.
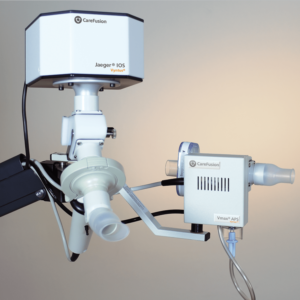
IOS Impulse Oscillometry
Tidal breathing analysis with Impulse Oscillometry (IOS) has demonstrated to be informative and differentiated in the early detection and follow up of pulmonary diseases like asthma, COPD and idiopathic pulmonary fibrosis. IOS is almost independent of patient cooperation and can test a larger patient range than spirometry alone, from children to adult to geriatric patients.
IOS is available as a stand-alone device combined with a spirometry measurement program (MasterScreen IOS and Vyntus IOS) or as an add-on module to the MasterScreen series.
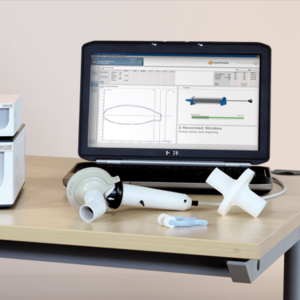
For all essential spirometry testing
The modular JAEGER Vyntus™ PNEUMO belongs to the Vyntus Family and is combinable with Vyntus APS for bronchial challenge testing and/or Vyntus IOS Impulse Oscillometry for measuring central and peripheral airway resistance.
Use your Vyntus Pneumo for all of the essential spirometry testing: Flow/Volume-loop (FVC), Slow Vital Capacity (SVC), Maximum Voluntary Ventilation (MVV), Pre and Post medication testing or therapy control.
Optionally, Vyntus Pneumo allows expanding your diagnostic possibilities with measurements like Rocc, P0.1, Pmax, SNIP, rhinomanometry and compliance. Vyntus Pneumo is enhanced by the workflow- and quality-driven SentrySuite™ Software.
The reliable, proven, accurate, heated Jaeger pneumotach has been selected as the measurement device of choice in hundreds of publications. Its excellent dynamic range allows for testing a broad population, from small children to athletes. Thousands of PFT labs profit daily from its high performance.