With POCcelerator® Data Management System, you can simplify your POCT workflows and dramatically reduce workload for your staff. Collect and review all patient results, quality controls, operators, reagents, and device data in a clear and structured format in order to comply with regulatory guidelines.
Maintain control of your POC Ecosystem™ Solution containing hundreds of devices, dozens of sites, and thousands of operators. Improve workflow, extend and enhance clinical capabilities, and improve the overall profitability of POC operations.
Open Connectivity
Connect over 220 POCT devices across 70+ manufacturers. Simplify your workflow and streamline your IT infrastructure by consolidating multiple middleware systems into one.
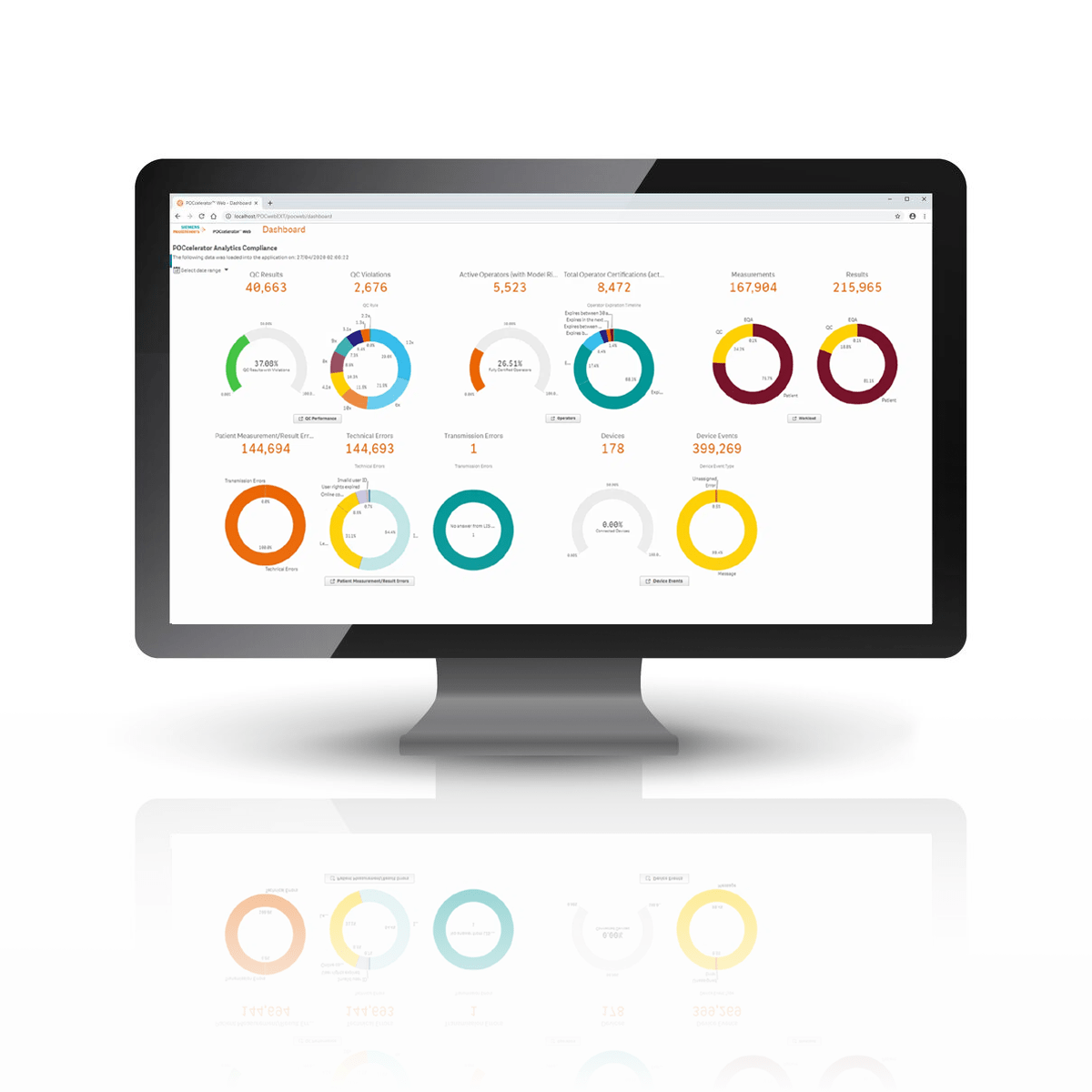
Recognize KPIs with POCcelerator Ci Module
At-a-glance metrics are available for Quality Control, Device Operators, Patient Results, and Device Performance. In-depth information is accessible with a few clicks.
Review trending reports
Analyze detailed reports
View your QC statistics for a visual representation of the selected criteria. Quickly assess any bias that may exist with different reagents.
Empower better care through deeper analytics and actionable insights
Every person, device, and POCT result must meet their respective standards of compliance. And you need to be able to monitor that compliance data and analyze it in real time to reduce risk and advance care. Data-driven, proactive, and efficient point-of-care testing is about more than quality control—it’s the gateway to better care.
Combining the most powerful release of the POCcelerator® Data Management System with in-depth, enhanced data analysis provided by the POCcelerator Ci module, delivers intelligent compliance to digitalize healthcare in ways previously unimaginable.
Interested in connecting only Siemens Healthineers devices? Ask your local sales representative about POCcelerator® SE Data Management System today!
Connect POCcelerator Data Management System to your Siemens Healthineers devices for maximum device performance
Download the ONE Solution infographic to see the enhanced features and functionality of your Siemens Healthineers devices when paired with POCcelerator System.

Atellica, Auto-Checks, CLINITEK Advantus, CLINITEK Status, DCA Vantage, epoc, Multistix, POCcelerator, RAPIDComm, RAPIDLab, RAPIDPoint, STIX, and all associated marks are trademarks – owned by or licensed to Siemens Healthcare Diagnostics Inc., or its affiliates. All other trademarks and brands are the property of their respective owners.
Service is essential to keep medical equipment operational and safe. Our Nordic service team consists of experienced service technicians stationed across the Nordic countries. Our service technicians are ready to take on your every service need.”
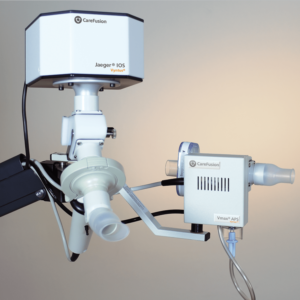
IOS Impulse Oscillometry
Tidal breathing analysis with Impulse Oscillometry (IOS) has demonstrated to be informative and differentiated in the early detection and follow up of pulmonary diseases like asthma, COPD and idiopathic pulmonary fibrosis. IOS is almost independent of patient cooperation and can test a larger patient range than spirometry alone, from children to adult to geriatric patients.
IOS is available as a stand-alone device combined with a spirometry measurement program (MasterScreen IOS and Vyntus IOS) or as an add-on module to the MasterScreen series.
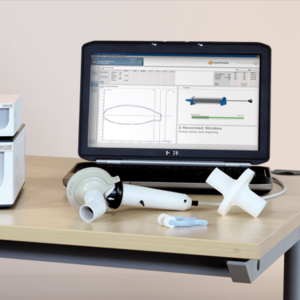
For all essential spirometry testing
The modular JAEGER Vyntus™ PNEUMO belongs to the Vyntus Family and is combinable with Vyntus APS for bronchial challenge testing and/or Vyntus IOS Impulse Oscillometry for measuring central and peripheral airway resistance.
Use your Vyntus Pneumo for all of the essential spirometry testing: Flow/Volume-loop (FVC), Slow Vital Capacity (SVC), Maximum Voluntary Ventilation (MVV), Pre and Post medication testing or therapy control.
Optionally, Vyntus Pneumo allows expanding your diagnostic possibilities with measurements like Rocc, P0.1, Pmax, SNIP, rhinomanometry and compliance. Vyntus Pneumo is enhanced by the workflow- and quality-driven SentrySuite™ Software.
The reliable, proven, accurate, heated Jaeger pneumotach has been selected as the measurement device of choice in hundreds of publications. Its excellent dynamic range allows for testing a broad population, from small children to athletes. Thousands of PFT labs profit daily from its high performance.

The dual-valved LiteAire MDI holding chamber: Collapsible, disposable paperboard design.
The LiteAire® collapsible MDI holding chamber is a unique MDI holding chamber and an innovative alternative. LiteAire’s unique dual-valved MDI holding chamber design delivers pop-up convenience and effective drug output at a fraction of the cost of most plastic holding chambers. In many clinical settings, the LiteAire can reduce costs by replacing existing rigid plastic holding chambers or inefficient spacers with a paperboard alternative. The unique design allows the LiteAire to be reused by a patient over multiple doses and meets and often exceeds the performance of plastic holding chambers.
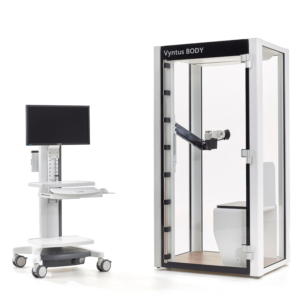
Vyntus™ BODY Plethysmograph
People come in all shapes and sizes, so we designed Vyntus BODY to accommodate the most patients without increasing the cabin footprint. Many enhancements were incorporated to make Vyntus BODY accessible and user friendly for both patients and technicians.
Vyntus BODY employs much of the same breakthrough technology found in the Vyntus ONE, but with a redesigned breathing circuit and our new Ultrasonic Sensor Technology.
In addition, Vyntus BODY is enhanced with additional innovation such as digital pressure compensation, which helps to reduce environmental influences and improves measurement accuracy.