Top-of-the-range FeNO and nNO measurement
NIOX VERO® is a portable non-invasive system, simple-to-use, point-of-care system that provides rapid standardized FeNO measurements. Simple and safe measurement of Nitric Oxide (NO) in human breath. Nitric Oxide is frequently increased in some inflammatory processes such as asthma.
Measuring the fractional NO concentration in expired breath (FeNO) provides the physician with means of evaluating an asthma patient’s response to anti-inflammatory therapy, as an adjunct to the established clinical and laboratory assessments in asthma.
NIOX system is used by healthcare professionals around the world to help improve asthma care, assisting:
NIOX VERO is portable system and can run on batteries. The products lifespan is 5 years or 15.000 measurements. It comes with pre-calibrated sensors and NO scrubber for NO-free air supply. Therefore, no daily calibration or maintenance is required.
NIOX VERO can also be connected to a PC, so the patient can be guided by animation either on the device screen or the connected PC screen.
NIOX VERO is suitable for children, 7–17 years, and adults 18 years and older. NIOX VERO can be operated in two exhalation modes, 10 seconds or 6 seconds. The 10-second test mode is for age 7 and up. The 6-second test mode is for ages 7–10 only when a 10-second test is not successful.

Service is essential to keep medical equipment operational and safe. Our Nordic service team consists of experienced service technicians stationed across the Nordic countries. Our service technicians are ready to take on your every service need.”
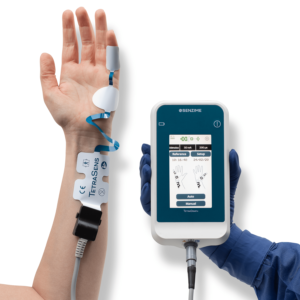
Delivering Confidence and Simplicity during Neuromuscular Blockade
TetraGraph is a quantitative neuromuscular transmission monitor based on electromyography (EMG). The TetraGraph monitor stimulates, measures, analyzes and displays muscle function in surgical patients receiving neuromuscular blocking agents (NMBAs). NMBAs are used in almost 50% of surgical cases. Inadequate reversal of NMBAs can lead to residual neuromuscular blockade (RNMB). RNMB delays recovery and can lead to life-threatening complications. To prevent complications, quantitative neuromuscular monitoring is rapidly becoming the standard of care when NMBAs are administered.
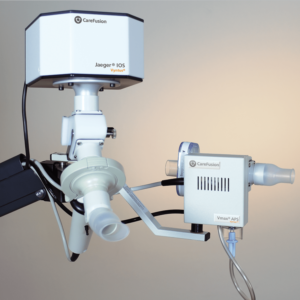
IOS Impulse Oscillometry
Tidal breathing analysis with Impulse Oscillometry (IOS) has demonstrated to be informative and differentiated in the early detection and follow up of pulmonary diseases like asthma, COPD and idiopathic pulmonary fibrosis. IOS is almost independent of patient cooperation and can test a larger patient range than spirometry alone, from children to adult to geriatric patients.
IOS is available as a stand-alone device combined with a spirometry measurement program (MasterScreen IOS and Vyntus IOS) or as an add-on module to the MasterScreen series.

Portable hospital spirometer
AioCare is a professional system to monitor and treat pulmonary diseases, consisting of a portable hospital-grade spirometer connected to a smartphone app and an online panel to access the results. The device enables a full spirometry test to be conducted in a doctor’s office, including a bronchial challenge workflow. If monitoring needs to go beyond the healthcare system, AioCare may be used for patients at home.
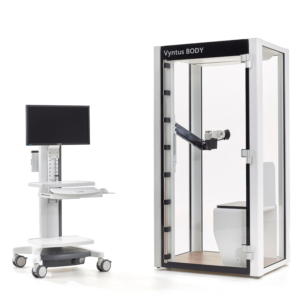
Vyntus™ BODY Plethysmograph
People come in all shapes and sizes, so we designed Vyntus BODY to accommodate the most patients without increasing the cabin footprint. Many enhancements were incorporated to make Vyntus BODY accessible and user friendly for both patients and technicians.
Vyntus BODY employs much of the same breakthrough technology found in the Vyntus ONE, but with a redesigned breathing circuit and our new Ultrasonic Sensor Technology.
In addition, Vyntus BODY is enhanced with additional innovation such as digital pressure compensation, which helps to reduce environmental influences and improves measurement accuracy.