Top-of-the-range FeNO and nNO measurement
NIOX VERO® is a portable non-invasive system, simple-to-use, point-of-care system that provides rapid standardized FeNO measurements. Simple and safe measurement of Nitric Oxide (NO) in human breath. Nitric Oxide is frequently increased in some inflammatory processes such as asthma.
Measuring the fractional NO concentration in expired breath (FeNO) provides the physician with means of evaluating an asthma patient’s response to anti-inflammatory therapy, as an adjunct to the established clinical and laboratory assessments in asthma.
NIOX system is used by healthcare professionals around the world to help improve asthma care, assisting:
NIOX VERO is portable system and can run on batteries. The products lifespan is 5 years or 15.000 measurements. It comes with pre-calibrated sensors and NO scrubber for NO-free air supply. Therefore, no daily calibration or maintenance is required.
NIOX VERO can also be connected to a PC, so the patient can be guided by animation either on the device screen or the connected PC screen.
NIOX VERO is suitable for children, 7–17 years, and adults 18 years and older. NIOX VERO can be operated in two exhalation modes, 10 seconds or 6 seconds. The 10-second test mode is for age 7 and up. The 6-second test mode is for ages 7–10 only when a 10-second test is not successful.

Service is essential to keep medical equipment operational and safe. Our Nordic service team consists of experienced service technicians stationed across the Nordic countries. Our service technicians are ready to take on your every service need.”

Untethered six-minute walk test
Vyntus™ WALK allows patients to perform an untethered six-minute walk test (6MWT) by wearing wireless sensors that communicate data to a technician-held tablet PC. Standardize testing to the recommended is 6MWT protocol, because consistent testing yields consistent data for comparison
The system is both patient- and technician-friendly and because we know a single sensor does not fit all clinical needs, comes with an array of easy-to-wear sensors. Because standardizing the complete testing process is critical to meaningful data for patient trending, Vyaire has integrated the complete ATS testing standards in the workflow-driven tablet software application.

Medisorb™ CO2 Absorbent
The Medisorb™ CO2 absorbent product line supports GE Healthcare anesthesia machines. Available in a wide range of sizes and styles, it is specifically designed to help change out absorbers simply. It is also available in a low-alkaline formulation, Medisorb™ EF.
As Medisorb™ soda lime becomes exhausted, it changes colour from white to violet. With the Medisorb™ EF EX, this colour change is longer lasting, thus helping to identify previously used absorbent. The Medisorb™ EF EX will also turn violet if desiccated. Note: Colour indication is not a definitive guide to remaining absorbent life and should always be used in conjunction with CO2 monitoring.
Medisorb™ product line:
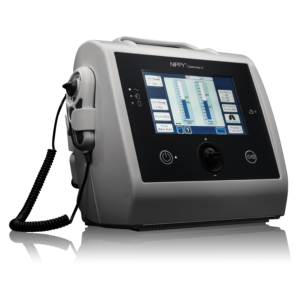
Versatile Airway Clearance
Introducing the Clearway 2 with TreatRepeat®. The second-generation MI-E device by Breas incorporates the latest technology to bring effective and accurate treatment for patients requiring airway clearance and cough assistance. TreatRepeat function allows clinicians to manually deliver different numbers of insufflations and exsufflations and then save the treatment they have just delivered to the device to be repeated automatically. Helping to improve accuracy and speed of titration and set-up.
Multiple mode options
Up to four treatment profiles can be saved to the device.The settings used in the manual and programmed modes are also saved to the device. This allows for up to 7 MI-E prescriptions to be saved. The treatment settings in the IPPB and NIV modes are also saved. The programmable mode enables the delivery of multiple insufflations as part of a cough cycle.
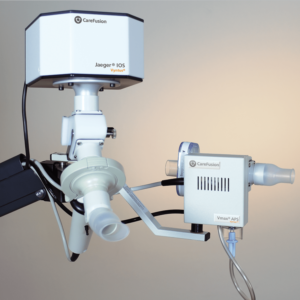
IOS Impulse Oscillometry
Tidal breathing analysis with Impulse Oscillometry (IOS) has demonstrated to be informative and differentiated in the early detection and follow up of pulmonary diseases like asthma, COPD and idiopathic pulmonary fibrosis. IOS is almost independent of patient cooperation and can test a larger patient range than spirometry alone, from children to adult to geriatric patients.
IOS is available as a stand-alone device combined with a spirometry measurement program (MasterScreen IOS and Vyntus IOS) or as an add-on module to the MasterScreen series.